Inflammation-induced preterm birth causes a shift in macrophage activity/morphology during cervix ripening

Marlena Ramanis '21
Abstract
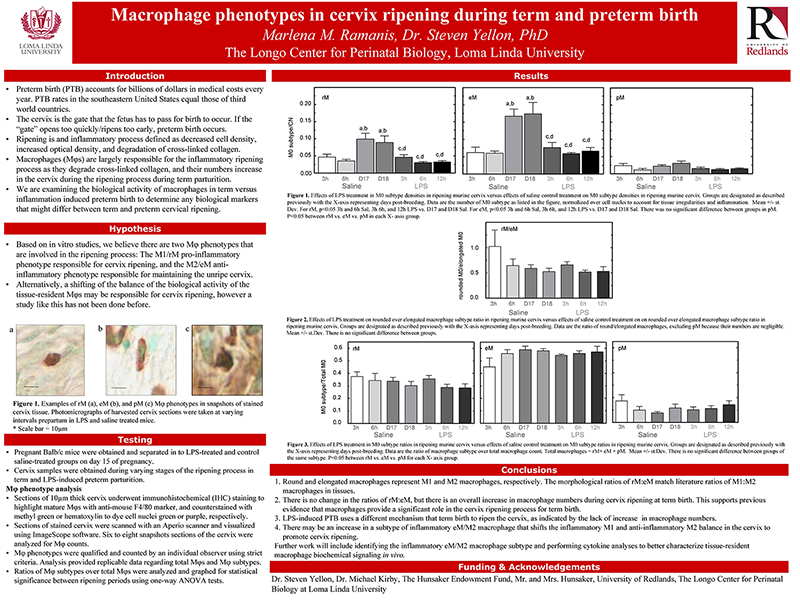
Cervix ripening is an inflammatory process that occurs well before labor at term and is characterized by reduced cell nuclei density, degradation of cross-linked collagen, and increased resident macrophages in the stroma. A well-establish model for inflammation induced cervix ripening and preterm birth (PTB) using the endotoxin lipopolysaccharide (LPS) indicates densities of cell nuclei (CN) and total macrophages (Mφs) do not change in cervix stroma from Sal- and LPS mice on D15. By D18 in Sal controls, CN density declines, resident Mφs increase (>2-fold), and cross-linked collagen is reduced. Sal control findings replicate previous results in other strains. By contrast, only cross-linked collagen/area declined by 12h versus 6h post-LPS - typical of inflammation in prepartum term ripening. The present study address the hypothesis that increased inflammatory activity by macrophage drives preterm ripening using the approach from in vitro studies that indicate macrophage subtypes M1 (inflammatory) or M2 (anti-inflammatory) phenotypes are, respectively shaped as round (rM) or elongated-spindled (eM). Accordingly, mice received saline (Sal) or the LPS (60µg, i.p. serotype) on day 15 postbreeding (D15.25 postbreeding; n=4-5/group) and P4 (100µg, s.c.) to sustain systemic concentrations. Postmortem, cervix sections from mice before (day 15.5) or on the day of preterm birth (day 16) were processed by immunohistochemistry and shape morphology of resident macrophages analyzed. In situ, >75% of Mφs were rM or eM (1:2 ratio) while others were polymorphic (pM). This provides strong evidence that shape may represent the two phenotypes (M1/M2) as suggested in the in vitro model, and can be used as a model for in vivo studies. In Sal controls on D18 vs D15, a 3-fold increase in density of all subtypes was found. Thus, prepartum term ripening was not solely due to the presumed inflammatory phenotype. In LPS-treated mice, no increase in macrophage numbers and no subtype changes were observed. This finding suggests that shape analysis is not predictive of the phenotypic changes that reflect an inflammatory drive during ripening. However, if macrophage shape reflects M1 and M2 phenotypic activity, finding also suggest that pathological and physiological cervix ripening differs in PTB and term birth due to the differences in macrophage densities in Sal and LPS- treated groups. Based on this evidence, inflammation-induced preterm cervix ripening may be due to a shift in the biological activity of the traditional M2 anti-inflammatory phenotype to a pro-inflammatory subtype of M2 macrophage in the cervix. Assessing the structural characteristics of cervix remodeling may unveil important biomarkers for ripening that predict the timing of birth and potential therapies to reduce or block pro-inflammatory macrophage activities that drive cervix ripening in women at risk of PTB.